|
|

|
|
Science Sparks @ ACTREC
|
 16 September 2024 16 September 2024 |
Vol. No. 13 ; Issue No.648 |
|
|
Publications
|
|
1. Dizendorf E, Chopra S, Mittal P, Gupta A, Nout R, Sturdza A, Chargari C, Tanderup K, Tharavichitkul E, Tatli H, Jeeva M, Jain J, Panda S, Upreti RR, Ghadi Y, Bhavke A, Kohle S, Bhajbhuje R, Agarwal JP. Gynecological brachytherapy hybrid training: The Tata Memorial Centre and BrachyAcademy experience. Brachytherapy.
2. Krishna R, Jadhav M, Sivasubramanian R, Sunkara G, Gota V, Niranjan V, Dhote V, Sapkal N, Rege NN, Sethi S, Medhi B, Kshirsagar N, Parmar D (2024). Advancing pediatric drug development in South Asia: Current landscape and vision for the future. Indian Journal of Pharmacology. 56(4): 285-289.
3. Thakur R, Kumar M, Kumar A, Joshi RK, Maheshwari D, Km AM, Venkataswamy M, Mohanty B, Chaudhari P, Mohan HK, Kumar P (2024). Synthesis, preclinical toxicity, and biodistribution of [18F]AVT-011 to assess the P-Glycoprotein function. Cancer Biotherapy and Radiopharmaceuticals.
4. Kumar P, Shanbhag N, Chaudhari P, Mohanty B, Thakur R, Gopalkrishnan MS (2024). Radiolabeling and preclinical evaluation of technetium-99m labeled colistin. Applied Radiation and Isotopes
5. Noronha V, Shah M, Pillai A, Menon N, Ramaswamy A, Ostwal V, Rao A, Kumar A, Dhekale R, Shetake A, Mahajan S, Daptardar A, Sonkusare L, Vagal M, Mahajan P, Timmanpyati S, Gota V, Niyogi D, Badwe R, Prabhash K (2024). Geriatric Assessment-guided therapy modification and outcomes in patients with non-metastatic gastroesophageal cancer: A retrospective cohort study. ESMO Gastrointestinal Oncology. 6: 100093.
|
|
|
|
|
Interesting Reads
|
|
Ascic E, Åkerström F, Sreekumar Nair M, Rosa A, Kurochkin I, Zimmermannova O, Catena X, Rotankova N, Veser C, Rudnik M, Ballocci T, Schärer T, Huang X, de Rosa Torres M, Renaud E, Velasco Santiago M, Met Ö, Askmyr D, Lindstedt M, Greiff L, Ligeon LA, Agarkova I, Svane IM, Pires CF, Rosa FF, Pereira CF (2024). In vivo dendritic cell reprogramming for cancer immunotherapy. Science.
|
|
|
Video of the Week
|
|
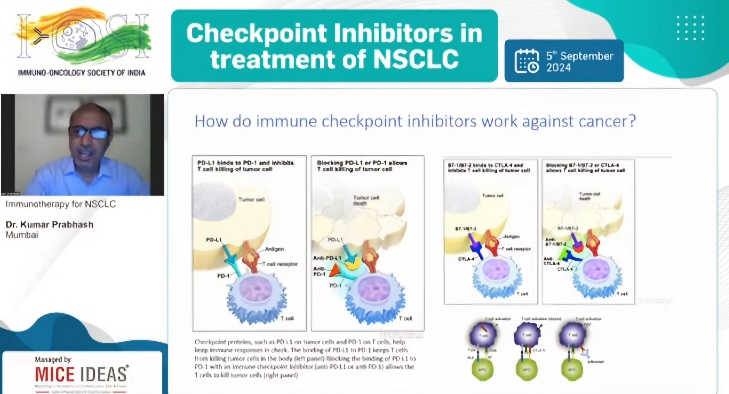
| Checkpoint Inhibitors in treatment of NSCLC |
|
| |
|
|
| |
|
|
|
|
Do You Know?
In 2004, Letrozole was approved by the FDA for the adjuvant treatment of early stage breast cancer after five years of tamoxifen therapy.
|
|
|
|
Cancer News
|
| |
|
ICMR signs agreements to advance first-in-human Phase 1 clinical trials
|
|
Deccan Herald, 14/09/2024
|
|
The ICMR Network for phase 1 Clinical Trials comprises four strategically located institutions across India, KEMH & GSMC- Mumbai, ACTREC-Navi Mumbai, SRM MCH&RC- Kattankulathur and PGIMER-Chandigarh supported by a central coordinating unit at ICMR headquarters, New Delhi...
|
| |
|
|
|
|
| |
|
|
|
|
|
|
|
|
2024 Advanced Centre for Treatment, Research and Education in Cancer (ACTREC)
|
|