|
|

|
|
Science Sparks @ ACTREC
|
 16 October 2023 16 October 2023 |
Vol. No. 12; Issue No. 600 |
|
|
|
|
|
|
Interesting Reads
|
|
Mossmann D, Müller C, Park S, Ryback B, Colombi M, Ritter N, Weißenberger D, Dazert E, Coto-Llerena M, Nuciforo S, Blukacz L, Ercan C, Jimenez V, Piscuoglio S, Bosch F, Terracciano LM, Sauer U, Heim MH, Hall MN (2023). Arginine reprograms metabolism in liver cancer via RBM39. Cell.
|
|
|
Video of the Week
|
|
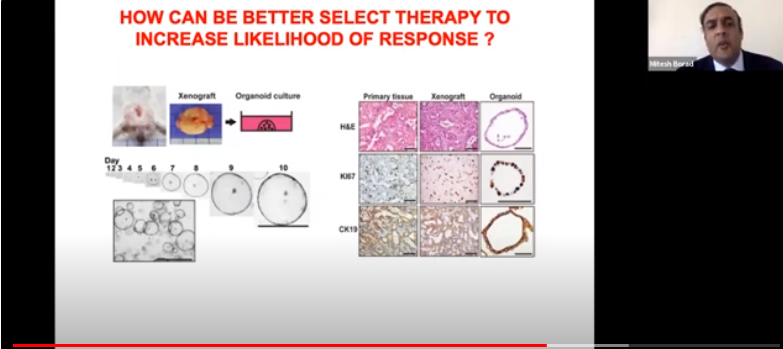
| Translational Dysequilibrium at the Bedside-Bench Interface |
|
| |
|
|
| |
|
|
|
|
Do You Know?
In 2009, FDA approved Cervarix, a second vaccine that protects against infection by the two HPV types that cause approximately 70% of all cases of cervical cancer worldwide.
|
|
|
|
Cancer News
|
| |
|
|
| |
|
|
|
India’s first cell therapy for cancer treatment receives regulatory approval
|
|
14 October 2023, Hindustan Times
|
|
The Central Drugs Standard Control Organization (CDSCO) has issued market authorisation to CAR-T (Chimeric Antigen Receptor-T) cell therapy, a breakthrough treatment for treating relapsed/refractory (r/r) B-cell lymphomas and leukaemia, paving the way for its commercial launch of indigenous NexCAR19 in the country. ImmunoACT, an IIT Bombay incubated company, developed the treatment. Both ImmunoACT and Tata Memorial Centre (TMC) collaborated to invent indigenous CAR-T Cell Therapy, a type of cancer immunotherapy treatment that uses immune cells called T cells that are genetically altered in a laboratory to enable them to locate and destroy cancer cells more effectively...
|
|
|
|
|
|
|
|
|
2019 Advanced Centre for Treatment, Research and Education in Cancer (ACTREC)
|
|
